Researchers Have Found A Way To Convert CO2 Into Jet Fuel (And Possibly Diesel)
Have you ever been out jogging in cold weather, (ha ha ha, no) watching your moist, hot exhales condense into clouds of alabaster steam and thought to yourself, hey, why can't we run airplanes on this shit? Well, good news! A bunch of scientists must be listening to your brainwaves via satellite, because a bunch of scientists from the University of Oxford (inventors of that comma) have figured out a way to do basically that: turn carbon dioxide into jet fuel. And, yes, they're using an organic combustion-synthesized iron-manganese-potassium catalyst, just like you imagined.
It's not hard to figure out why this could prove to be so valuable; jet engines produce lots of CO2 as exhaust, so being able to repurpose that waste gas and turn it back into fuel transforms a once linear path of waste into a cycle of re-use. The scientists even made a little diagram to explain this:
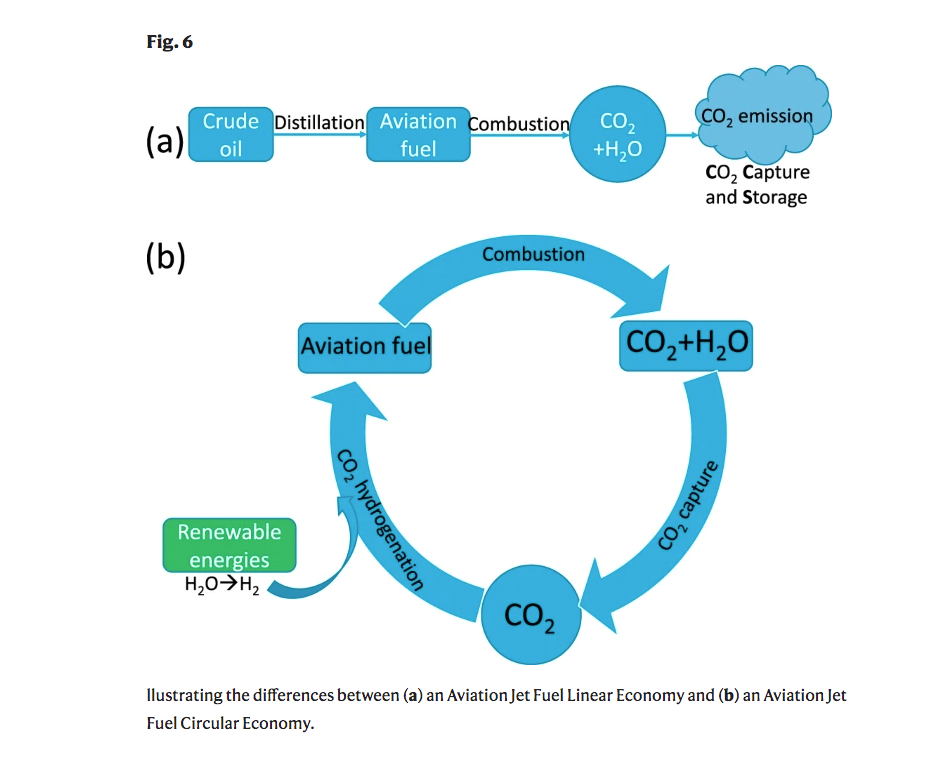
Even though jet fuel is full of carbon, getting the carbon in CO2 to become the sort of carbon needed in jet fuel is not easy. From the report on the breakthrough in Nature:
Nevertheless, the activation of CO2 is extremely challenging; CO2 is a fully oxidized, thermodynamically stable and chemically inert molecule. Furthermore, hydrocarbon synthesis via the hydrogenation of CO2 usually favours the formation of short-chain, rather than desirable long-chain, hydrocarbons. Hence most of the research in this area have focused on the selective hydrogenation of CO2 to CH4, the oxygenates, CH3OH, HCOOH, and light olefins (C2–C4 olefins)22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41, There have been limited studies on producing liquid hydrocarbons of molecularity C5+42,43,44.
That's all just basically saying this is hard. What the Oxford team came up with uses a a chemical reaction known as organic combustion, sometimes called the Solution Combustion Method, which is used to assemble a catalyst made from iron, manganese and potassium.
Incredibly, the researchers found that commercial sugar and flour could be used as fuels to make the catalyst:
Finally, we have also examined commercial sugar and flour powders as possible fuels in the catalyst preparation process. Catalysts prepared with these fuels also showed high CO2 hydrogenation activity and jet fuel range hydrocarbon selectivity. The catalytic performance for CO2 hydrogenation of catalysts prepared with different fuels are shown in Supplementary Figs. 10–22.
The catalyst helps add the needed hydrogen atoms to the CO2, which is crucial to processing it into jet fuel.
Somehow, this diagram explains it — or at least part of it — but likely more effectively to people smarter than me:
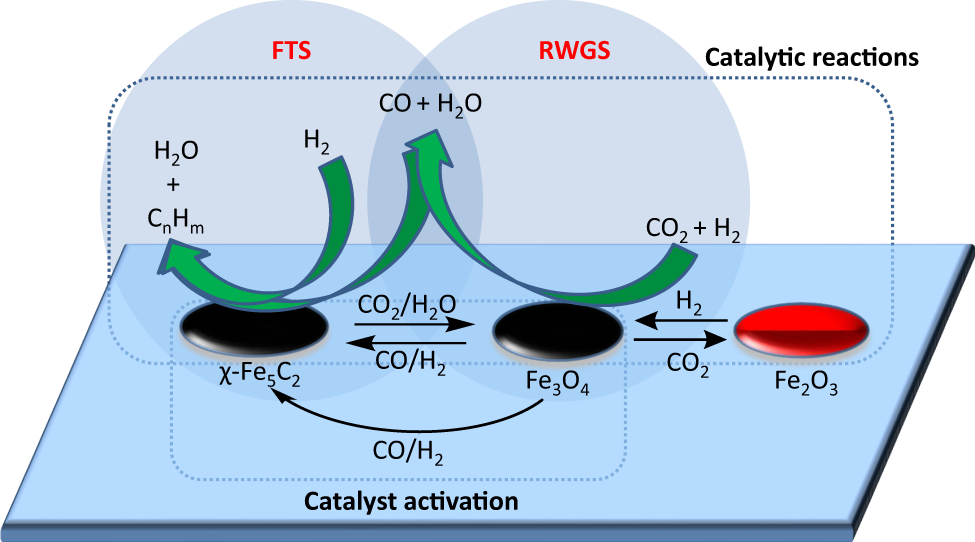
If you're really feeling smart, here, have a look at this which shows how he CO2 and H2 conversion and product selectivity was calculated:
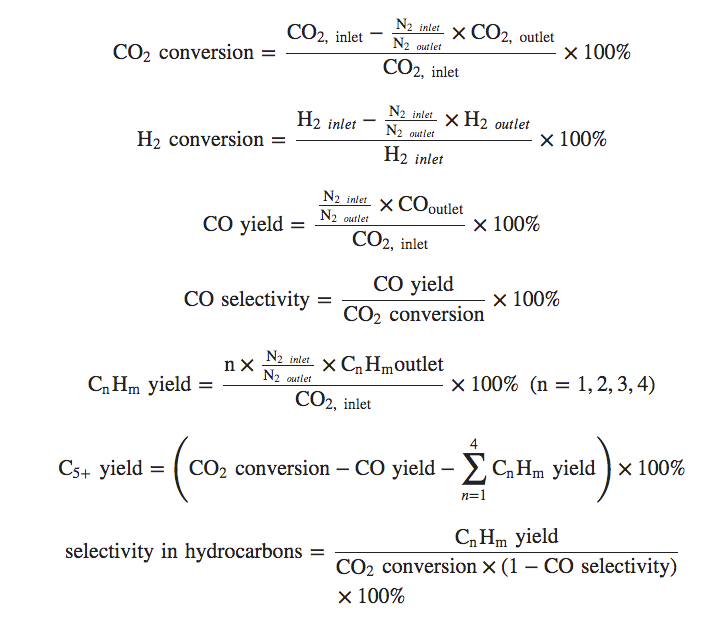
I don't know really how to read that, but I like how impressively sciencey it looked, so I stuck it in here.
The study doesn't address this specifically, but what could potentially be another use for this process is the generation of diesel fuels from carbon dioxide since Jet A fuel is very close to diesel. In fact, you can run Jet A right in your diesel car or truck, though it has less lubricant, so if you try this you should really add some supplemental lubrication.
If this method proves scalable (which, based on the relative simplicity and efficiency of the method, it should) perhaps it could be used to produce lubricant-added diesel fuel as well, which is also in huge demand.
Hopefully, this will actually, um, take off, and all my panting and wheezing will help send you off on your next vacation.
